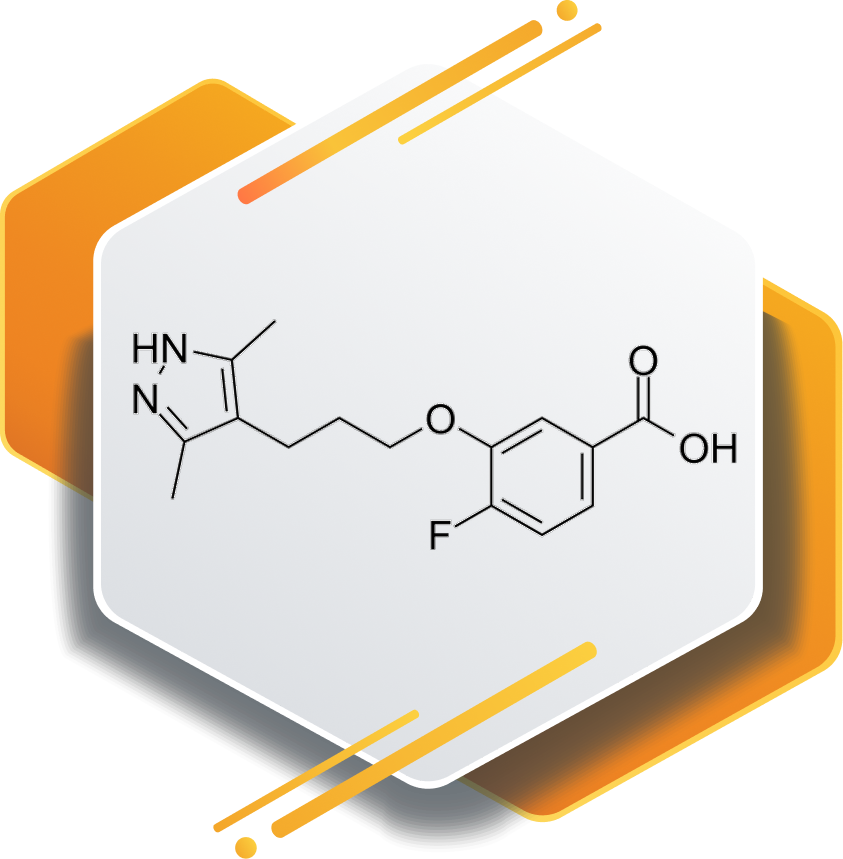
Kinetic stabilizer of transthyretin (TTR)
Acoramidis is an orally active small molecule designed to stabilize transthyretin. It is widely researched for its role in amyloid-related disorders and supports development of targeted, disease-modifying therapeutic solutions.
Acoramidis received regulatory approval from the U.S. Food and Drug Administration (FDA) in November 2024 and the European Commission in February 2025 for the treatment of transthyretin amyloid cardiomyopathy (ATTR-CM)
Mechanism of Action
Acoramidis is an approved drug used for the treatment of transthyretin (TTR) amyloidosis, a serious condition caused by the misfolding and deposition of transthyretin protein in various organs, especially the heart. Acoramidis works by stabilizing the transthyretin tetramer, preventing its breakdown into monomers that can aggregate and form harmful amyloid fibrils.
Acoramidis offers an important therapeutic option for patients with both hereditary and wild-type TTR amyloidosis, aiming to improve cardiac function, quality of life, and overall survival. It is positioned as a competitor to earlier TTR stabilizers like Tafamidis, with clinical studies showing promising efficacy and safety profiles.
Acoramidis Improves Survival & Functional Status in ATTR-CM
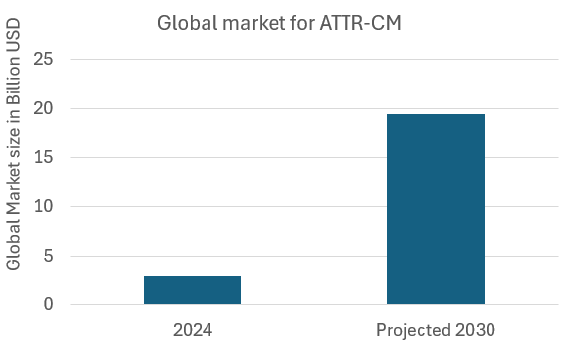
Market reality and projection
The global ATTR-CM treatment market is expected to grow from USD 2.91 billion in 2025 to USD 19.37 billion by 2032, exhibiting a CAGR of 31.1%.
Let's Discuss Your Requirement
What we can offer
Acoramidis for test and Development activities.
Process Impurities.
Improved process for Acoramidis manufacturing.
Detailed tech-pack for Acoramidis manufacturing, including Analytical Method, and working reference standard of intermediates and impurities.
