TRPM8 Calcium channel Agonist (TRPM8)
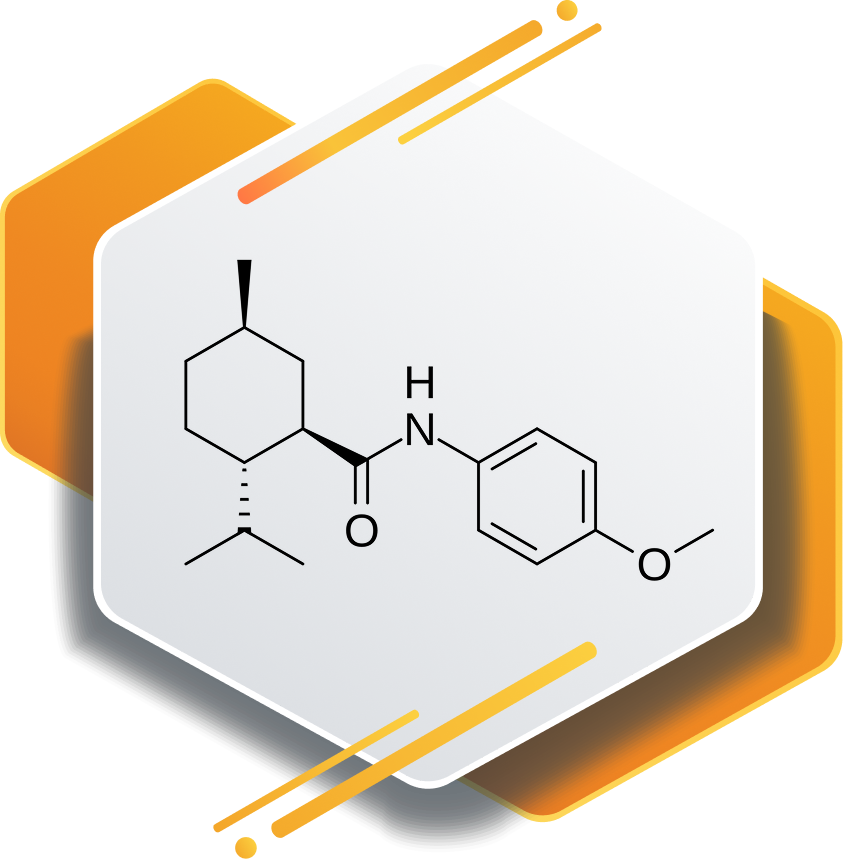
Acoltremon is a selective small-molecule modulator studied for neurological and cognitive research. It plays a key role in exploring innovative treatments by targeting specific neural pathways linked to brain function and balance.
Acoltremon received regulatory approval from the U.S. Food and Drug Administration (FDA) in May 2025 for the treatment of Dry Eye Syndrome.
Mechanism of Action
Acoltremon acts as an agonist of the transient receptor potential melastatin 8 (TRPM8) thermoreceptor, a cold-sensing ion channel found on corneal sensory nerves. By activating TRPM8, acoltremon stimulates trigeminal nerve signaling, which leads to a rapid increase in natural basal tear production. This neuromodulatory effect directly addresses tear deficiency, a key factor in dry eye disease.
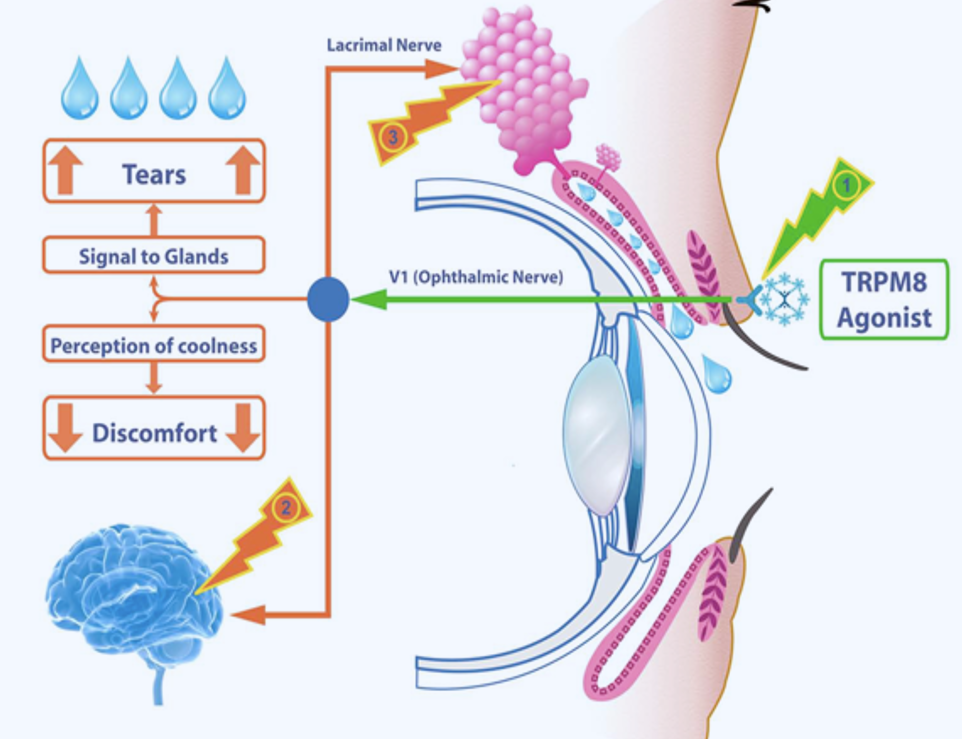
Acoltremon offers an important therapeutic option for conditions caused by inadequate or poor-quality tears, helping to reduce discomfort, improve vision clarity, and protect the ocular surface.
Market reality and projection
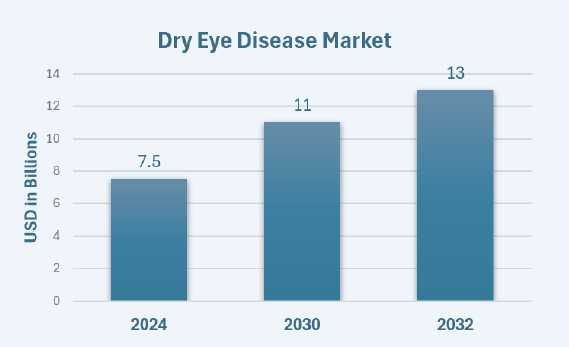
- The global DED treatment market was valued at ~USD 6.3–7.5 billion in 2024 and is projected to reach ~USD 9.2–11.0 billion by 2030. Longer-range views place the market near USD ~13.0 billion by 2032.
- The market is expected to expand at a compound annual growth rate (CAGR) of 7.5%.
- Key drivers of market growth include an aging population, rising screen time (especially in urban centres), harsh winters/low indoor humidity and urban air pollution, a growing burden of autoimmune and endocrine disorders (e.g., rheumatoid arthritis, thyroid disease), steadily increasing healthcare expenditure with expanding private care/pharmacy access, and improved awareness and diagnosis through ophthalmology/optometry networks.