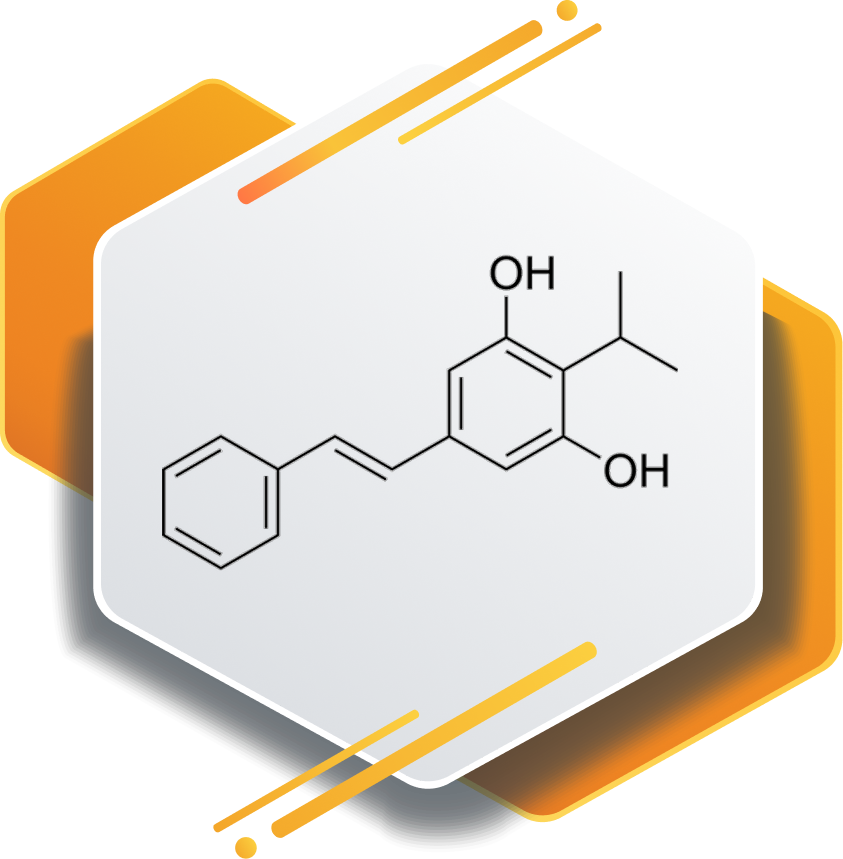
First-in-class Aryl hydrocarbon Receptor (AhR)- Agonist
Tapinarof is a novel aryl hydrocarbon receptor agonist used in dermatology research. It supports anti-inflammatory and skin barrier pathways, making it valuable for developing therapies for chronic inflammatory skin disorders.
Approved in the United States in May 2022 for the treatment of Psoriasis and Atopic Dermattis.
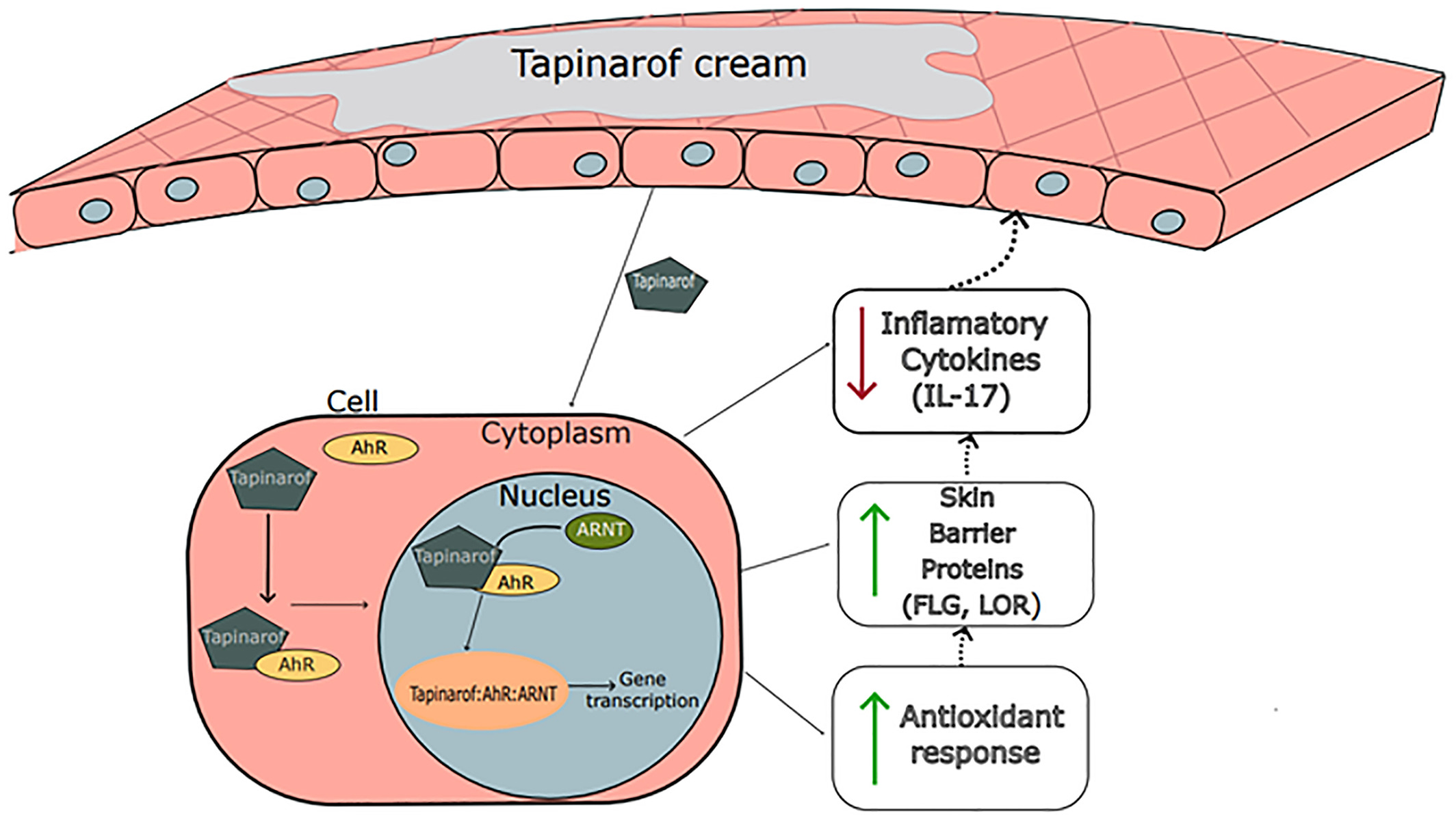
Mechanism of Action
Tapinarof acts as an aryl hydrocarbon receptor (AhR) agonist. By selectively activatng AhR, a ligand-activated transcription factor expressed in skin cells, tapinarof modulates gene expression involved in skin barrier function, inflammation, and oxidative stress. This mechanism contributes to its anti-inflammatory and immune regulating properties, making it effective in treating chronic skin disorders such as plaque psoriasis and atopic dermatitis. Unlike traditional immunosuppressants or corticosteroids, tapinarof offers a non-steroidal approach, potentially reducing long-term safety concerns while delivering durable therapeutic benefits.
Psoriasis Market Overview & Forecast:
In 2024, the psoriasis treatment market was valued at USD 20.3 billion across the top 7 markets, and is expected to grow to USD 38.4 billion by 2035, at a CAGR of ~5.96% from 2025 to 2035.
Atopic Dermatitis Market Overview & Forecast:
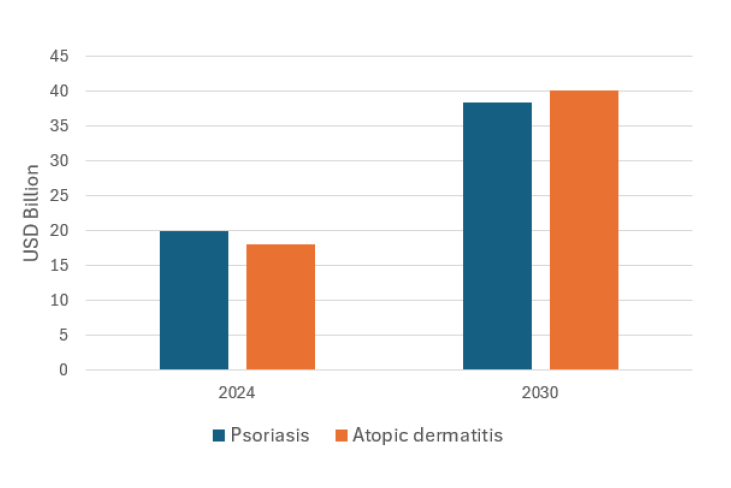
Tapinarof Market Projection:
Let's Discuss Your Requirement
What we can offer
Tapinarof for test and Development activities.
High-purity Tapinarof provided for research, formulation, validation, scale-up, stability, and development programs.
Process Impurities.
Tapinarof impurities supplied for method development, validation, profiling, quality control, and regulatory use.
Tapinarof API (Bulk).
Novel improved process for Tapinarof manufacturing.
Detailed tech-pack for Tapinarof manufacturing, including Analytical Method, and working reference standard of intermediates and impurities.
Exclusive right for a novel improved process.
We are in the process of applying for an Indian provisional patent for the Improved process of Tapinarof Manufacturing.
